

The value of l (azimuthal quantum number) will be equal to 2.

(The most stable one would be the 2 D 1 / 2 state, according to Hund's rules for less-than-half-filled orbitals with the same S and the same L. Number of Radial nodesnl1 Number of angularnodesl Where lAzimuthalquantumnumbernprincipalquantumnumber For 4d orbital n4 andl2 In4d orbital the number. Solution The value of n for a 4 d orbital will always remain 4. In this case, it would give a term symbol of 2 D 1 / 2 , 2 D 3 / 2 and 2 D 5 / 2 The notation is : Principal quantum number (n) Azimuthal quantum number (l) Magnetic quantum number (m l) Spin quantum number (m s) 1. In that case, the electron is, by default, If it happens to be a 4 d 1 configuration, for example, then one of five orbitals are filled ( ( d x 2 − y 2, d z 2 , d x , d x z , d y z )) with one electron. Electron holding capacity of shells For example, n 1 for K orbit. The electron holding capacity of each orbit is 2n 2.
#4d orbital quantum numbers full
ĭepending on how full the orbital is, m s varies. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. Thus, its m 1 varies as 0, ± 1, ± 2 and the orbital has projection above the plane and below the plane. n = 4 specifies the energy level ,and l specifices the orbitl's shape.
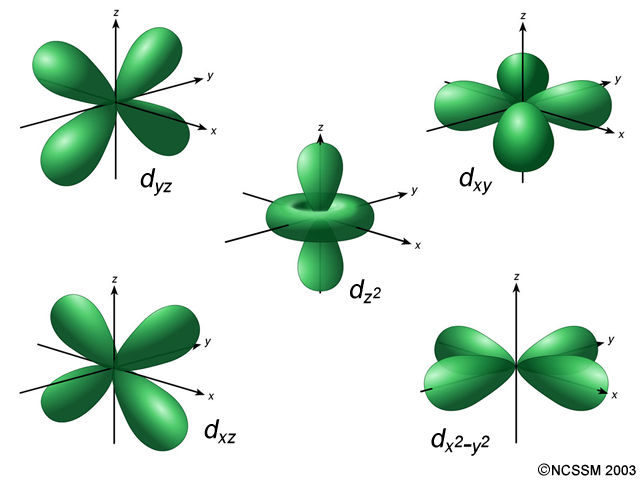
The subshell number is represented by the azimuthal quantum number l. The value of l depends on the value of n such that l 0, 1. SECONDARY QUANTUM NUMBER (l ) - Represents the energy sublevel, or type of orbital, occupied by the electron. It is always a positive integer, that is n 1, 2, 3. The four quantum numbers of interest are n (principal quantum number), l (angular momentum) m 1 (magnetic, and m s (spin )Ī generic 4 d z 2 orbital has n = 4 and l = 2. Quantum Numbers: The shell number of an electron is represented by the principal quantum number n. PRINCIPAL QUANTUM NUMBER (n) - Represents the main energy level, or shell, occupied by an electron.


 0 kommentar(er)
0 kommentar(er)
